Patients with primary progressive MS treated continuously with ocrelizumab saw greater benefit in regard to measures of disease progression compared with patients who switched from placebo during. Results have been announced as positive for the.
 Biomedicines Free Full Text Diagnosis And Management Of Progressive Multiple Sclerosis Html
Biomedicines Free Full Text Diagnosis And Management Of Progressive Multiple Sclerosis Html
The drug which is delivered via intravenous infusion every 6 months following a loading dose had updates to its drug label with new safety information earlier this year in January.

Ocrelizumab secondary progressive ms. It is a disease-modifying drug DMD made from monoclonal antibodies proteins. Ocrevus ocrelizumab is a disease modifying drug DMD that is approved for relapsing remitting and for primary progressive MS. OCREVUS ocrelizumab Results for PPMS Progressive MS Find out why OCREVUS may be the right choice for treating your primary progressive multiple sclerosis PPMS.
OCREVUS was studied against a placebo. You take Ocrevus as an intravenous infusion every six months. A placebo is a substance or treatment that has no active.
It is administered by intravenous infusion. Relapsing forms of multiple sclerosis MS to include clinically isolated syndrome relapsing-remitting disease and active secondary progressive disease in adults. It is not known if OCREVUS is safe and effective in children.
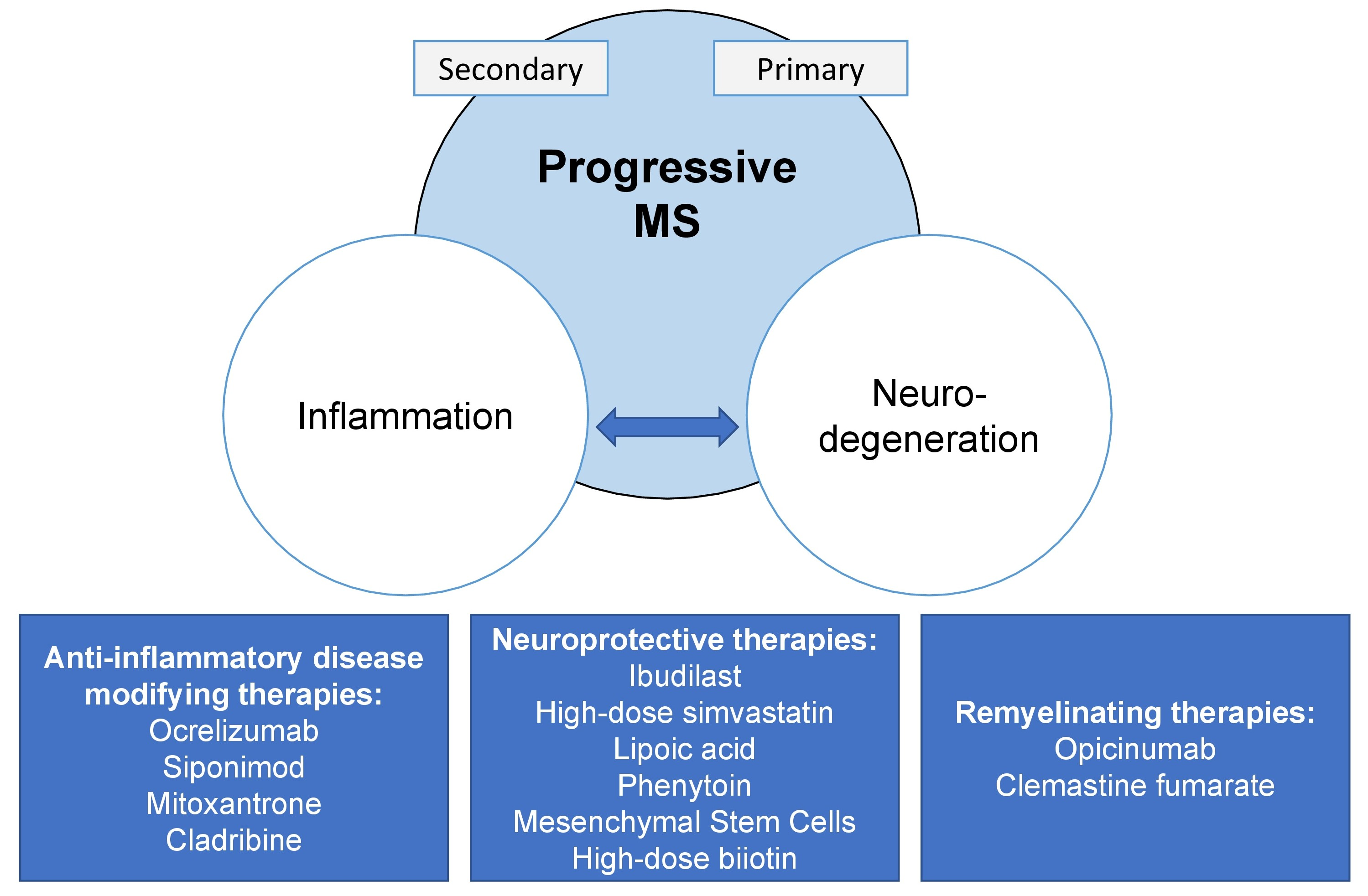
The pathophysiology of progressive multiple sclerosis includes acute inflammatory lesion activity chronic inflammation and neurodegeneration1 The exact relationship between these components remains unclear. Ocrevus may also be used for purposes not listed in this medication guide. Ocrevus is a CD20-directed cytolytic antibody indicated for the treatment of patients with relapsing secondary progressive or primary progressive forms of multiple sclerosis MS as well as those with clinically isolated syndrome CIS.
Food and Drug Administration FDA approved ocrelizumab for the treatment of relapsing forms of MS including secondary progressive MS with relapses and primary progressive MS based on the pivotal phase III data. In the US ocrelizumab is indicated for the treatment of relapsing forms of multiple sclerosis MS to include clinically isolated syndrome relapsing-remitting disease and active secondary progressive disease in adults or for the treatment of primary progressive MS in adults. Primary progressive multiple sclerosis accounts for 10 to 15 of the overall population with multiple sclerosis.
Ocrevus has twice-yearly six-monthly dosing and is the first and only therapy approved for relapsing multiple sclerosis RMS including RRMS and active or relapsing secondary progressive MS SPMS in addition to clinically isolated syndrome CIS in the US and primary progressive MS PPMS. Ocrelizumab was approved for the treatment of relapsing forms of MS including clinically isolated syndrome as well as secondary progressive and primary progressive disease in 2017. Food and Drug Administration has approved Ocrevus ocrelizumab - Genentech a member of the Roche Group for use in the treatment of primary progressive MS and relapsing MS making it the first therapy for the treatment of primary progressive multiple sclerosis.
Home Ocrevus ocrelizumab for Primary Progressive and Relapsing MS Ocrevus generic name ocrelizumab is a humanized monoclonal antibody that was approved bythe US. Its licence allows it to be used for early primary progressive MS too. Roche has reported encouraging data with Ocrevus a humanised monoclonal antibody designed to selectively target CD20-positive B cells ocrelizumab in RRMS and SPMS and also primary progressive MS an early-onset form of the disease in which there is no relapsingremitting stage and that also has no approved therapies.
Will ocrelizumab help people with secondary progressive MS. Ocrelizumab is used to treat active relapsing MS. Despite success in the development of disease-modifying therapies for relapsing-remitting multiple sclerosis treatment of progressive multiple sclerosis has proven more difficult.
NICE approves Ocrevus ocrelizumab for primary progressive MS Published on 09 May 2019 The MS Trust is delighted that Ocrevus ocrelizumab has been approved by NICE for NHS treatment of early inflammatory primary progressive MS. The OPERA I and OPERA II studies enrolled a total of 1656 people with relapsing forms of MS ie relapsing-remitting MS and secondary-progressive MS with relapses across 307 sites in 40 countries. 1 The course of this disease differs from those of relapsingremitting and.
Food and Drug. It reduces the number of relapses by about two thirds 70 compared to taking placebo. Primary progressive MS in adults.
Ocrevus will be available as a first-line treatment which means that there are no recommendations in the approved labeling for people to try other MS therapies before taking it. See results and understand how OCREVUS was evaluated in a clinical trial. Ocrelizumab is an investigational humanised monoclonal antibody designed to selectively target CD20-positive B cells.
Its now recommended to be used on the NHS across the UK to treat both these types of MS. So far the clinical trials of ocrelizumab have involved people with relapsing forms of MS relapsing-remitting MS and people with secondary progressive MS who were experiencing relapses and primary progressive MS. Ocrevus is used to treat primary progressive multiple sclerosis and relapsing forms of multiple sclerosis in adults including clinically isolated syndrome relapsing-remitting disease and active secondary progressive disease.