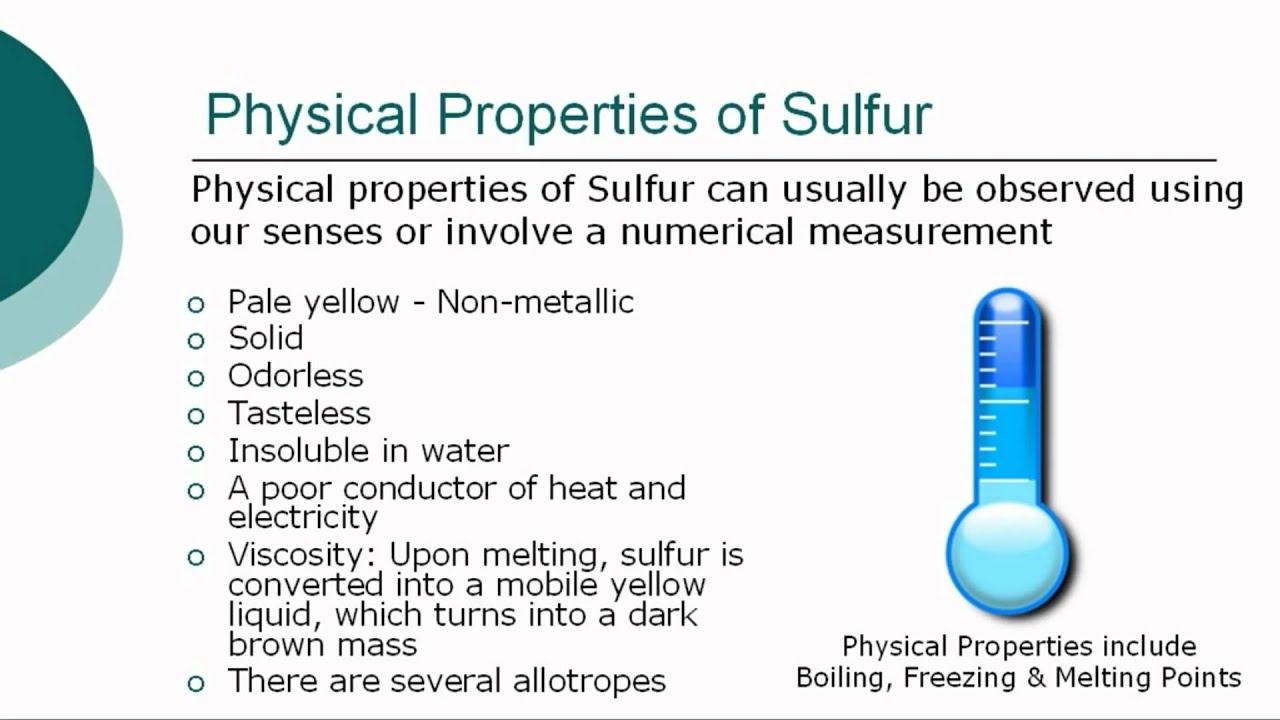
Physical properties characterizing sulfur Rhombic a-S and monoclinic v-S molecules contain 8 sulfur atoms which are connected in a closed loop with single covalent bonds. The Physical properties of Sulfur are the characteristics that can be observed without changing the substance into another substance.
 Chemical And Physical Properties Of Sulphur Dioxide And Sulphur Trioxide Springerprofessional De
Chemical And Physical Properties Of Sulphur Dioxide And Sulphur Trioxide Springerprofessional De
Physical Properties of Sulphuric Acid H2SO4 Sulphuric acid is a colorless viscous heavy liquid having a density of 184 gmL at 298 K.

Physical properties of sulphur. Sulfur S also spelled sulphur nonmetallic chemical element belonging to the oxygen group Group 16 VIa of the periodic table one of the most reactive of the elements. Sulfur is insoluble in water but soluble in carbon disulfide and to a lesser extent in other nonpolar organic solvents such as benzene and toluene. Pure sulfur is a tasteless odourless brittle solid that is pale yellow in colour a poor conductor of electricity and insoluble in water.
Sulfur S is an element that can never be overlooked. Elemental sulfur is a bright yellow crystalline solid at room temperature. Physical Properties of Sulphur Physical Properties of Sulphur in solid state does not conduct electricity appreciably and so forms a convenient insulating material for some purposes although it is more frequently used only as an ingredient of insulating compounds or mixtures.
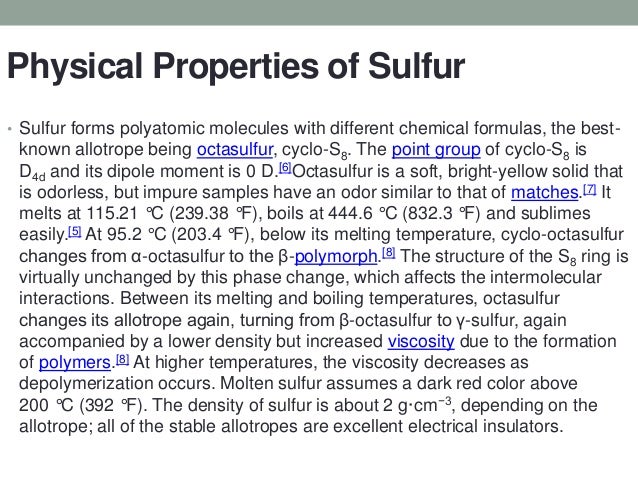
This sulfur is 995 to 999 pure and requires no purification for most uses. Under normal conditions sulfur atoms form cyclic octatomic molecules with a chemical formula S 8. It is abundant multivalent and nonmetallic.
Under normal conditions sulfur has a rhombic modification. Rhombic monoclinic polymeric and others. It is used in the production of phosphate fertilizers in oil refineries and mineral extraction.
In the periodic table sulfur is found in group 16. Sulfur was mined near Mount Etna in Sicily and used for bleaching cloth and preserving wine both of which involved burning it to form sulfur dioxide and allowing this to be absorbed by wet clothes or the grape juice. The air forces the liquid sulfur mixed with air to flow up through the outlet pipe.
S8 or octa-sulfur is one of the most popular molecules of sulfur. It is a yellow solid crystalline substance with a density of 207 g cm 3. It is abundant multivalent and nonmetallic.
Physical properties are usually those that can be observed using our senses such as color luster freezing point boiling point melting point density hardness and odor. Sulphur is a chemical element with the symbol S and atomic number 16. This family in the upper right of the periodic table is called the Chalcogen family or more commonly known as the oxygen family.
The Frasch process is used to mine sulfur from underground deposits. Sulfur appears in a number of different allotropic modifications. It also spelled sulphur is a chemical element with the symbol S and atomic number 16.
Though the data given in this chapter are available in literature the practical application of the remarkable physical as well as chemical properties of sulphur dioxide and sulphur trioxide has been experienced and applied on large scale only recently. It is non-metal and is obtained as a byproduct after the production of natural gas. Despite such figures S 2 is rare with S 4 and S 6 being more common.
The first and the second ionization energies of sulfur are 9996 and 2252 kJmol respectively. It is a soft solid that is odourless and has a bright yellow colour. What are the Physical Properties of Sulfur.
Sulfur is also used as a fungicide and in the manufacture of gunpowder. The fourth and sixth ionization energies are 4556 and 84958 kJmol. The physical properties of sulfur are that it is atomic number 16 and has an atomic weight of 3206.
Sulphuric acid freezes at 104C and boils at 317C with a slight High viscosity and high boiling point of sulphuric acid is due to extensive hydrogen bonding in it. Sulphur usually occurs as a pale yellow brittle crystalline solid. Sulfur Cozzodisi Mine Agrigento Province Sicily Italy.
Sulfur can be found in the air in many different forms. Sulfur Chemical Properties Physical and Chemical Properties. Chemical properties health and environmental effects of sulphur.
Sulfur is an odorless tasteless light yellow solid. It can cause irritations of the eyes and the throat with animals when the uptake takes place through inhalation of sulfur in the gaseous phase. Physical Properties of Sulphur Sulfur or sulphur can form various polyatomic molecules.
Physical Properties Sulfur is a non-metal found on the periodic table and occupies group number 16. Sulfur in British English. Sulfuric acid H 2 SO 4 is a highly produced industrial chemical worldwide.
It is a reactive element that given favorable circumstances combines with all other elements except gases gold and platinum. Sulfur is the tenth most common element by mass in the universe and the fifth most common on Earth. In colour it is bright yellow and it has an extremely bad odour like rotten eggs.
Some of the most important physical properties of Sulphur are. It has a faint characteristic smell but no taste 4. Sulphur is highly soluble in carbon disulphide and sparingly soluble alcohol and ether and etc.
Transferring the mixture to large settling vats allows the solid sulfur to separate upon cooling. Sulfur is mentioned 15 times in the Bible and was best known for destroying Sodom and GomorrahIt was also known to the ancient Greeks and burnt as a fumigant.
 Properties And Uses Of Sulphur Dioxide Definition Examples Diagrams
Properties And Uses Of Sulphur Dioxide Definition Examples Diagrams
 Sulfur Alchetron The Free Social Encyclopedia
Sulfur Alchetron The Free Social Encyclopedia
 1 Summary Of The Chemical And Physical Properties Of Sulfur Dioxide Download Table
1 Summary Of The Chemical And Physical Properties Of Sulfur Dioxide Download Table
 Sulfur Ppt Video Online Download
Sulfur Ppt Video Online Download
 Table 1 From A Review Of The Chemistry Pesticide Use And Environmental Fate Of Sulfur Dioxide As Used In California Semantic Scholar
Table 1 From A Review Of The Chemistry Pesticide Use And Environmental Fate Of Sulfur Dioxide As Used In California Semantic Scholar
 So3 Sulfur Trioxide Structure Molecular Mass Properties And Uses
So3 Sulfur Trioxide Structure Molecular Mass Properties And Uses
 The Sulfur In Sulphur Dichloride Formally Is In The Oxidation State Ppt Download
The Sulfur In Sulphur Dichloride Formally Is In The Oxidation State Ppt Download
 Sulfur Definition Element Symbol Uses Facts Britannica
Sulfur Definition Element Symbol Uses Facts Britannica
 Physicochemical Properties Of Sulfur Mustard Download Table
Physicochemical Properties Of Sulfur Mustard Download Table
Difference Between Sulfur And Sulfur Dioxide Definition Physical And Chemical Properties Uses
 Physical Properties My Favorite Element Sulfur
Physical Properties My Favorite Element Sulfur